Prosigna
Breast Cancer Recurrence Risk
Prosigna
Breast Cancer Recurrence Risk
Advantages
SYNLAB GROUP
Guaranteed by the experience of the absolute European leader in laboratory diagnostics.
COMPLETE
- Validated in two clinical studies that evaluated more than 2,400 postmenopausal women with early-stage breast cancer.
- Approved by the regulatory agencies of the United States (FDA) and Europe (EMA) as a prognostic tool in early breast cancer;
- Provides the assessment of recurrence risk over up to 10 years and the intrinsic subtype.
Extra Information
DOCUMENTATION – Available on the SYNLAB Direct for clients
- Informed Consent;
- Clinical Questionnaire;
- Medical Request.
PREPARATION
- Fasting is not necessary for the exam.
Additional Information
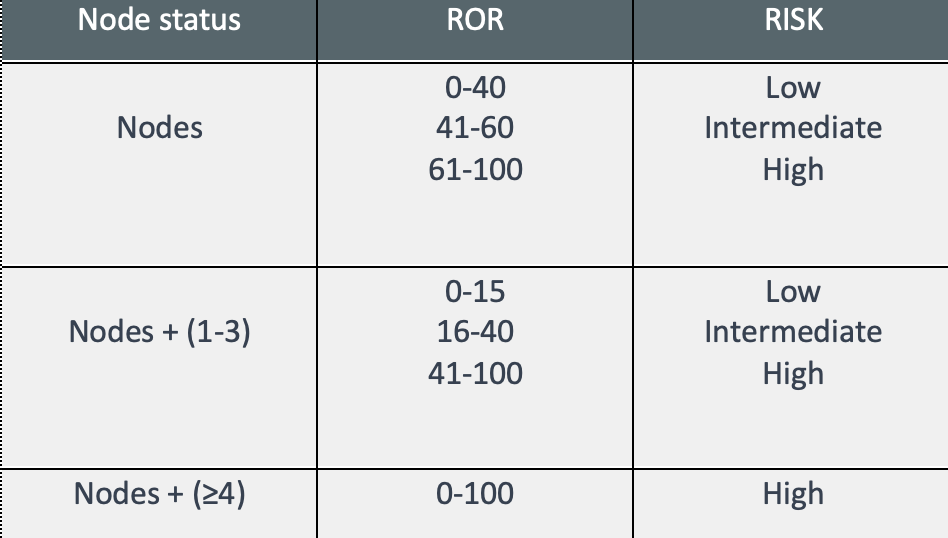
The report includes different parameters:
- Lymph node involvement and Intrinsic subtype – risk classification and information for therapeutic guidelines.
- ROR– numerical value from 0 to 100 related to the 10-year recurrence risk;
- Risk classification: Establishes the risk of recurrence in 10 years, classifying into three possible groups: Low risk, intermediate risk, and high risk.