Personalization in Physical Activity: Discover the Impact of DNA
Regular physical activity is essential for a healthy lifestyle, playing…
Continue reading

Mental disorders represent an increasing challenge to global public health, affecting millions of people across all age groups. Conditions like depression, anxiety, and bipolar disorders are among the leading causes of disability worldwide, significantly impacting quality of life and social interactions.
In recent years, research has explored the connection between the gut microbiome and mental health, suggesting that the gut flora plays a crucial role in regulating the brain and behavior, opening new perspectives for the prevention and treatment of these conditions.
Keep reading to discover how the gut microbiome and depression are related, as well as the influence of this ecosystem on overall mental health.
The gut microbiota is a dynamic ecosystem that develops alongside the host organism, influenced by the physiological conditions of this environment. The microbiome, in turn, refers to the complete set of genes of a microorganism community, representing the total genetic material that makes up the microbiota. This includes the DNA of bacteria, viruses, fungi, and other microorganisms that inhabit the gut.
In this blog article, we discuss the key differences and details of the gut microbiota and microbiome.
A diverse gut microbiome is directly linked to good health. This concept of diversity refers to the presence of a wide variety of species coexisting in balance. The more heterogeneous the microbiome, the greater the variety of functions performed by the microorganisms, contributing to the proper functioning of the human body.
In the human body, there are almost as many human cells as microbial ones. When analyzing the microbiome, it is important to remember that each of the thousands of species present has its own genome. Therefore, the variety of microbial genes in our body far exceeds that of our human genes. These microbial genes produce substances that can impact our health either positively or negatively.
An increasing number of diseases are associated with alterations in the gut microbiome. The mechanism behind this relationship stems from the fact that microorganisms can produce different biologically active metabolites, which may play a role in disease states.
Various mental and developmental disorders have been linked to the gut microbiome. In addition to an altered microbiome, many individuals with these conditions suffer from gastrointestinal issues, such as irritable bowel syndrome. Previously, it was believed that depression and anxiety caused or contributed to these problems. However, recent evidence suggests the opposite: gut inflammation may influence the development of mental disorders, such as depression and anxiety.
The connection between the gut and the brain was identified when it was observed that patients with mental or mood disorders, such as depression, anxiety, and schizophrenia, also had imbalances in their gut microbiome (1). Furthermore, people with irritable bowel syndrome or inflammatory bowel diseases often experienced neurological conditions (2), giving rise to the concept of the “microbiome-gut-brain axis” (3).
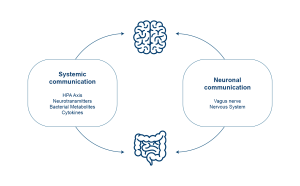
The gut-brain axis is a bidirectional communication network that integrates gut functions with the brain’s cognitive and emotional centers. It involves the central nervous system, the autonomic and enteric nervous systems, as well as the neuroendocrine, enteroendocrine, and neuroimmune systems (4, 5).
This axis plays a crucial role in mediating the effects of genetic and environmental factors on brain development and function and has been associated with the etiology of several psychiatric disorders.
The communication system is supported by various pathways, including the vagus nerve, the intestinal endocrine system, the immune system, and microbial metabolites. Short-chain fatty acids and neurotransmitters like GABA, which are produced and consumed by gut microorganisms, are examples of metabolites that influence this axis (6).
Metabolites can reach the brain through the vagus nerve and the bloodstream. The vagus nerve connects the brain to the colon and other organs, allowing gut molecules to influence mood, while the brain can also send signals back to the gut.
In this way, the gut-brain axis not only integrates intestinal functions with brain activities but also demonstrates how the balance of the gut microbiome can impact mental and emotional health.
Depression is one of the most common mental disorders, yet its causes remain partially unknown, and its diagnosis is complex.
Recent studies have demonstrated characteristic variations in the gut microbiome of individuals with depression, where some of the involved bacteria produce metabolites like glutamate, butyrate, serotonin, and gamma-aminobutyric acid (GABA), substances with potent neurological action. These substances can exert their effects through the vagus nerve, which connects the gut to the brain, influencing mood and depression (7).
The relationship between the gut microbiome and depression involves several complex mechanisms, such as:
These mechanisms show how the gut microbiome may play an important role in regulating mood and mental health, suggesting that interventions aimed at restoring a healthy microbiome could have therapeutic potential for depression.
The relationship between the gut microbiome and mental health is bidirectional, meaning that changes in the composition or functionality of the gut microbiome lead to changes in the production or availability of essential microbial products (18).
Similarly, emotional states such as stress activate the production of hormones like cortisol, increasing gut permeability and altering the composition of the gut microbiota, favoring the proliferation of pro-inflammatory species (19).
Stress is known to increase gut permeability, thus providing bacteria an opportunity to translocate through the intestinal mucosa and directly access immune cells and neurons in the central nervous system. This is a potential pathway through which the microbiota may influence the CNS via the immune system and the enteric nervous system in the presence of stress.
The delicate balance between the human microbiome and the development of psychopathologies is particularly interesting given how easily the microbiome can be altered by external factors, such as diet (20), antimicrobial exposure (21), or disrupted sleep patterns (22), for example.
Recent studies have demonstrated that diet is one of the most important modulators of the gut-brain-microbiome axis, as it allows modifications to the microbiome’s composition and regulates the production of key microbial metabolites (23). This is possible through dietary interventions based on individual microbiomes, targeting the modulation of microbial groups or functionalities, thus promoting good mental health.
Each person’s gut microbiome is unique, formed by the microorganisms we have been exposed to throughout life, influenced by antibiotic use, genetics, lifestyle, and diet (24).
Various studies show that diet is a key factor in the composition and functioning of the gut microbiome. This happens because nutrients interact directly with microorganisms, favoring or inhibiting their growth depending on their ability to extract energy from food (25).
Among the nutrients most utilized by the microbiome are indigestible carbohydrates, called glycans, primarily of plant origin, where studies suggest that a diet rich in these compounds can help maintain a balanced microbiome, as well as promote health and increase bacterial diversity (26).
Another important nutritional strategy is the regular consumption of fermented foods, which expose the gut to beneficial microorganisms. A recent study indicates that a diet rich in fermented foods not only increases microbial diversity but may also reduce inflammatory markers in the blood (27).
The challenge lies in how to put these recommendations into practice. What truly makes a difference to health is maintaining a balanced diet in the long term, without focusing solely on specific nutrients (28).
Moreover, diet personalization based on each person’s microbiome is essential, as everyone responds differently to food. The ultimate goal is to develop personalized diets that improve or adjust specific microbial groups, exploring the unique potential of each microbiome to promote health.
An imbalanced microbiome, known as dysbiosis, occurs when there is a disruption in the composition or function of gut microorganisms. This imbalance can be corrected with the consumption of prebiotics and/or probiotics. Prebiotics are fiber-rich foods that promote the growth of beneficial bacteria in the gut.
By adding different types of prebiotics to the diet and reducing foods that favor potentially harmful microorganisms (such as additives and excess proteins), we can rebalance the microbiome, providing the necessary nutrients for healthy bacteria to thrive.
In addition to diet, lifestyle plays a key role in maintaining gut microbiome balance. Reducing stress, ensuring good sleep quality, and exercising are factors that directly contribute to this balance.
To effectively restore the microbiome, it is essential to understand its current state and how it is impacting your health. For this, a test with high coverage and resolution, providing detailed information about the behavior of gut microorganisms, is indispensable.
SYNLAB offers the MyBiome test, an exclusive test that analyzes the gut microbiome through massive sequencing (shotgun metagenomics), providing a complete reading of the genome of intestinal microorganisms. MyBiome offers:
MyBiome provides detailed information on gut microorganisms and their impact on health, with direct clinical application.
MyBiome is especially recommended for:
Accurate and up-to-date testing is essential for more accurate diagnoses and better treatment guidance. SYNLAB is here to help.
We offer diagnostic solutions with rigorous quality control to the companies, patients, and doctors we serve. We have been in Brazil for over 10 years, operate in 36 countries across three continents, and are leaders in service provision in Europe.
Contact the SYNLAB team and learn more about the available tests.
References
Regular physical activity is essential for a healthy lifestyle, playing…
Continue reading
The Small Intestinal Bacterial Overgrowth (SIBO) syndrome was identified several…
Continue reading
Mental disorders represent an increasing challenge to global public health,…
Continue reading