FG Cardio Hypertension
Pharmacogenetics of Arterial Hypertension
Why undergoing this examination?
Hypertension is often an asymptomatic condition that can lead to serious and lethal complications if not treated in time. In this scenario, chronic hypertension is the most important modifiable risk factor for the development of both cardiovascular and cerebrovascular and renal diseases. In the pharmacological treatment of arterial hypertension, a series of medications are used to counteract the mechanisms of its pathophysiology: alpha and beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEIs), among others. However, despite recent advances in pharmacological treatment, not all patients with arterial hypertension achieve the expected blood pressure levels with medication, and many must combine two or more drugs. In this context, Pharmacogenetics, which studies the genetic influence on the response to drug metabolism among different individuals, can be a key investigation in the search for success in prognosis and patient treatment.
What is this exam?
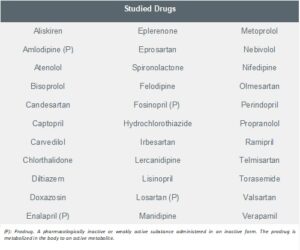
The FG Cardio Hypertension pharmacogenetic panel evaluates metabolizing enzymes and targets involved in the effect and toxicity of various drugs used in the treatment of arterial hypertension. The analysis provides relevant information on the 33 most commonly used drugs, based on the study of 30 genetic variants of genes responsible for the expression of enzymes involved in the metabolism of these drugs. The genes include: CYP3A4, NPPA, CACNA1C, LDLR, CYP2D6, CYP2C9, AGTR1, ADRB2, ACE/ECA, ADD1, APOB, ADRB1, SLCO1B1, CYP1A2, CYP2C19, GNB3.
For whom is it indicated?
- Patients with hypertension who want to personalize treatment based on their genetic profile;
- Patients undergoing pharmacological treatment who do not achieve the expected results.
Technology
Next-generation sequencing (NGS)
FG Cardio Hypertension
Pharmacogenetics of Arterial Hypertension
Hypertension is often an asymptomatic condition that can lead to serious and lethal complications if not treated in time. In this scenario, chronic hypertension is the most important modifiable risk factor for the development of both cardiovascular and cerebrovascular and renal diseases. In the pharmacological treatment of arterial hypertension, a series of medications are used to counteract the mechanisms of its pathophysiology: alpha and beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEIs), among others. However, despite recent advances in pharmacological treatment, not all patients with arterial hypertension achieve the expected blood pressure levels with medication, and many must combine two or more drugs. In this context, Pharmacogenetics, which studies the genetic influence on the response to drug metabolism among different individuals, can be a key investigation in the search for success in prognosis and patient treatment.
The FG Cardio Hypertension pharmacogenetic panel evaluates metabolizing enzymes and targets involved in the effect and toxicity of various drugs used in the treatment of arterial hypertension. The analysis provides relevant information on the 33 most commonly used drugs, based on the study of 30 genetic variants of genes responsible for the expression of enzymes involved in the metabolism of these drugs. The genes include: CYP3A4, NPPA, CACNA1C, LDLR, CYP2D6, CYP2C9, AGTR1, ADRB2, ACE/ECA, ADD1, APOB, ADRB1, SLCO1B1, CYP1A2, CYP2C19, GNB3.
- Patients with hypertension who want to personalize treatment based on their genetic profile;
- Patients undergoing pharmacological treatment who do not achieve the expected results.
Next-generation sequencing (NGS)
Advantages
SYNLAB GROUP
Guaranteed by the experience of the absolute European leader in laboratory diagnostics.
COMPLETE
Detailed report where the results will suggest individualized behaviors, aiding in prognosis to provide greater effectiveness in treatment and a significant reduction in adverse reactions.
Additional Information
DOCUMENTATION – Available on the SYNLAB Direct for clients
- Informed consent;
- Clinical questionnaire;
- Medical request.
PREPARATION
- Fasting is not necessary for the test.
Additional Information

Delivery Time
22 business days

Sample Type
5mL of total blood in EDTA