Genotyping KIR + HLA-C
Genetic immunological assessment in reproductive success
Why undergoing this examination?
During the first trimester of gestation, trophoblastic cells from the blastocyst invade the wall of the maternal uterus, contributing to the formation of the placenta. A defect in the placental origin process can lead to various disorders, such as premature births, preeclampsia, fetal growth restriction, or gestational losses. Studies have shown that part of the regulation of the placental formation process occurs under the influence of local immune recognition. Trophoblastic cells express high levels of HLA-C, which are recognized by KIR (killer immunoglobulin-like receptor) and uNK (uterine Natural Killer cells). KIRs are expressed from a highly polymorphic gene family. These genes are grouped and classified into haplotype A (predominantly inhibitory KIRs) and haplotype B (predominantly activating KIRs). The presence of activating KIRs (haplotype B) provides protection against pregnancy complications, while their absence (haplotype A) increases the risk of complications. The balance between activation and inhibition allows the release of cytokines that favor trophoblastic placental invasion.
What is this exam?
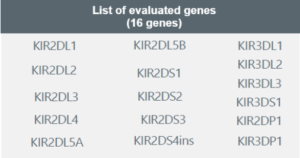
The Genotyping KIR + HLA-C test is a genetic test that determines the individual’s haplotype based on the 16 described genes and performs molecular typing of human leukocyte antigen alleles (HLA-C class I) at the allelic group level. The result allows the assessment of the risk of pregnancy complications based on the present haplotype, enabling treatment direction for a better reproductive response.
For whom is it indicated?
The Genotyping KIR and HLA-C test is primarily recommended for cases of reproductive failures.
Technology
Molecular Hybridization – PCR SSP + Multiplex PCR SSO.
Genotyping KIR + HLA-C
Genetic immunological assessment in reproductive success
During the first trimester of gestation, trophoblastic cells from the blastocyst invade the wall of the maternal uterus, contributing to the formation of the placenta. A defect in the placental origin process can lead to various disorders, such as premature births, preeclampsia, fetal growth restriction, or gestational losses. Studies have shown that part of the regulation of the placental formation process occurs under the influence of local immune recognition. Trophoblastic cells express high levels of HLA-C, which are recognized by KIR (killer immunoglobulin-like receptor) and uNK (uterine Natural Killer cells). KIRs are expressed from a highly polymorphic gene family. These genes are grouped and classified into haplotype A (predominantly inhibitory KIRs) and haplotype B (predominantly activating KIRs). The presence of activating KIRs (haplotype B) provides protection against pregnancy complications, while their absence (haplotype A) increases the risk of complications. The balance between activation and inhibition allows the release of cytokines that favor trophoblastic placental invasion.
The Genotyping KIR + HLA-C test is a genetic test that determines the individual’s haplotype based on the 16 described genes and performs molecular typing of human leukocyte antigen alleles (HLA-C class I) at the allelic group level. The result allows the assessment of the risk of pregnancy complications based on the present haplotype, enabling treatment direction for a better reproductive response.
The Genotyping KIR and HLA-C test is primarily recommended for cases of reproductive failures.
Molecular Hybridization – PCR SSP + Multiplex PCR SSO.
Advantages
SYNLAB GROUP
Guaranteed by the experience of the absolute European leader in laboratory diagnostics.
COMPLETE
Report with objective results and detailed interpretation.
Extra Information
DOCUMENTATION – Available on the SYNLAB Direct for clients
- Informed Consent;
- Clinical Questionnaire;
- Medical prescription.
PREPARATION
- Fasting is not necessary for the test.
Additional Information
See below information that contributes to a better understanding of the test:

Delivery Time
9 business days

Sample Type
10mL whole blood in EDTA












