Prosigna
Breast Cancer Recurrence Risk
Why undergoing this examination?
In recent years, the understanding of breast cancer has evolved significantly, now considered a complex and heterogeneous pathology. Therefore, distinct factors must be considered to determine the patient’s prognosis. Women with early-stage breast cancer expressing estrogen receptors usually have a good prognosis. However, in this population, about 50% of recurrences occur after five years of diagnosis. An imminent need in the management of estrogen receptor-positive breast cancer is to identify women at increased risk for late recurrence.
What is this exam?
Prosigna (PAM50) was developed based on a PAM50 genetic signature that provides the patient’s risk of recurrence information (ROR – Risk Of Recurrence) based on three factors: tumor size, intrinsic molecular subtype, and tumor proliferation status.
For whom is it indicated?
Postmenopausal women who have undergone mastectomy due to breast cancer and have the following characteristics:
- Hormone receptor-positive, node-negative, stage I and II breast cancer;
- Hormone receptor-positive, node-positive (1-3, 4 or more positive nodes), stage II or IIIA breast cancer.
Technology
nCounter Dx Analysis System
Prosigna
Breast Cancer Recurrence Risk
In recent years, the understanding of breast cancer has evolved significantly, now considered a complex and heterogeneous pathology. Therefore, distinct factors must be considered to determine the patient’s prognosis. Women with early-stage breast cancer expressing estrogen receptors usually have a good prognosis. However, in this population, about 50% of recurrences occur after five years of diagnosis. An imminent need in the management of estrogen receptor-positive breast cancer is to identify women at increased risk for late recurrence.
Prosigna (PAM50) was developed based on a PAM50 genetic signature that provides the patient’s risk of recurrence information (ROR – Risk Of Recurrence) based on three factors: tumor size, intrinsic molecular subtype, and tumor proliferation status.
Postmenopausal women who have undergone mastectomy due to breast cancer and have the following characteristics:
- Hormone receptor-positive, node-negative, stage I and II breast cancer;
- Hormone receptor-positive, node-positive (1-3, 4 or more positive nodes), stage II or IIIA breast cancer.
nCounter Dx Analysis System
Advantages
SYNLAB GROUP
Guaranteed by the experience of the absolute European leader in laboratory diagnostics.
COMPLETE
- Validated in two clinical studies that evaluated more than 2,400 postmenopausal women with early-stage breast cancer.
- Approved by the regulatory agencies of the United States (FDA) and Europe (EMA) as a prognostic tool in early breast cancer;
- Provides the assessment of recurrence risk over up to 10 years and the intrinsic subtype.
Extra Information
DOCUMENTATION – Available on the SYNLAB Direct for clients
- Informed Consent;
- Clinical Questionnaire;
- Medical Request.
PREPARATION
- Fasting is not necessary for the exam.
Additional Information
The report includes different parameters:
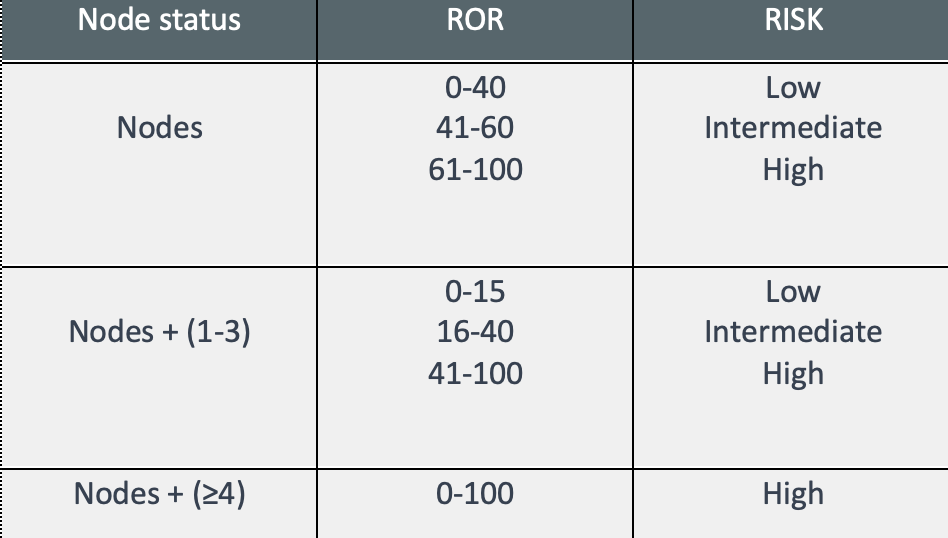
- Lymph node involvement and Intrinsic subtype – risk classification and information for therapeutic guidelines.
- ROR– numerical value from 0 to 100 related to the 10-year recurrence risk;
- Risk classification: Establishes the risk of recurrence in 10 years, classifying into three possible groups: Low risk, intermediate risk, and high risk.

Delivery Time
9 business days

Sample Type
Fragment of tumor tissue in a paraffin block (consult material preparation instructions).